Metastatic Colorectal Cancer (mCRC)
7
In combination with intravenous fluorouracil-based chemotherapy for first- or second-line treatment of patients with metastatic colorectal cancer (mCRC), and in combination with fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy for second-line treatment of patients with mCRC who have progressed on a first-line bevacizumab product-containing regimen.
Limitations of Use: Alymsys® is not indicated for adjuvant treatment of colon cancer
First-Line Non-Squamous Non small Cell Lung Cancer (NSCLC)
7
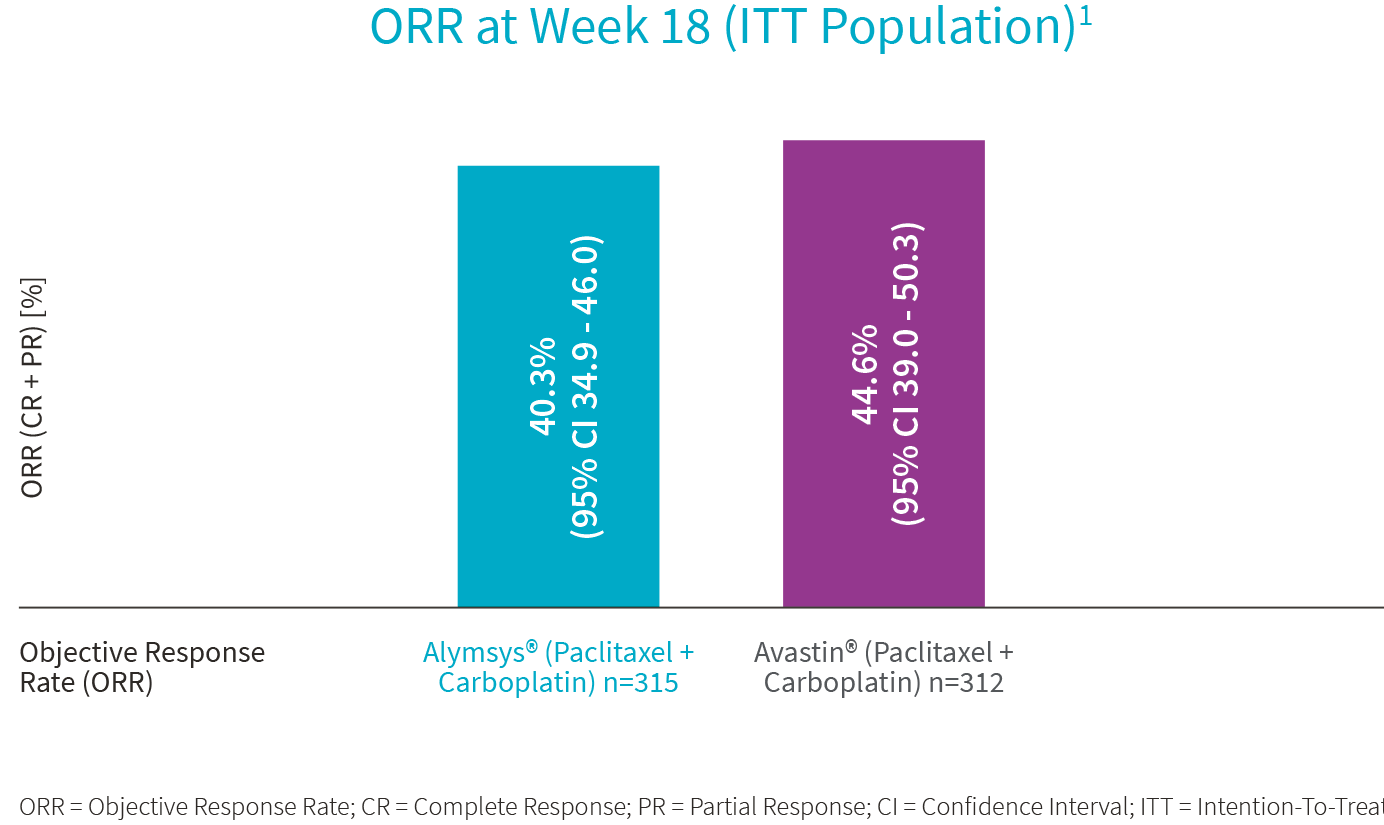
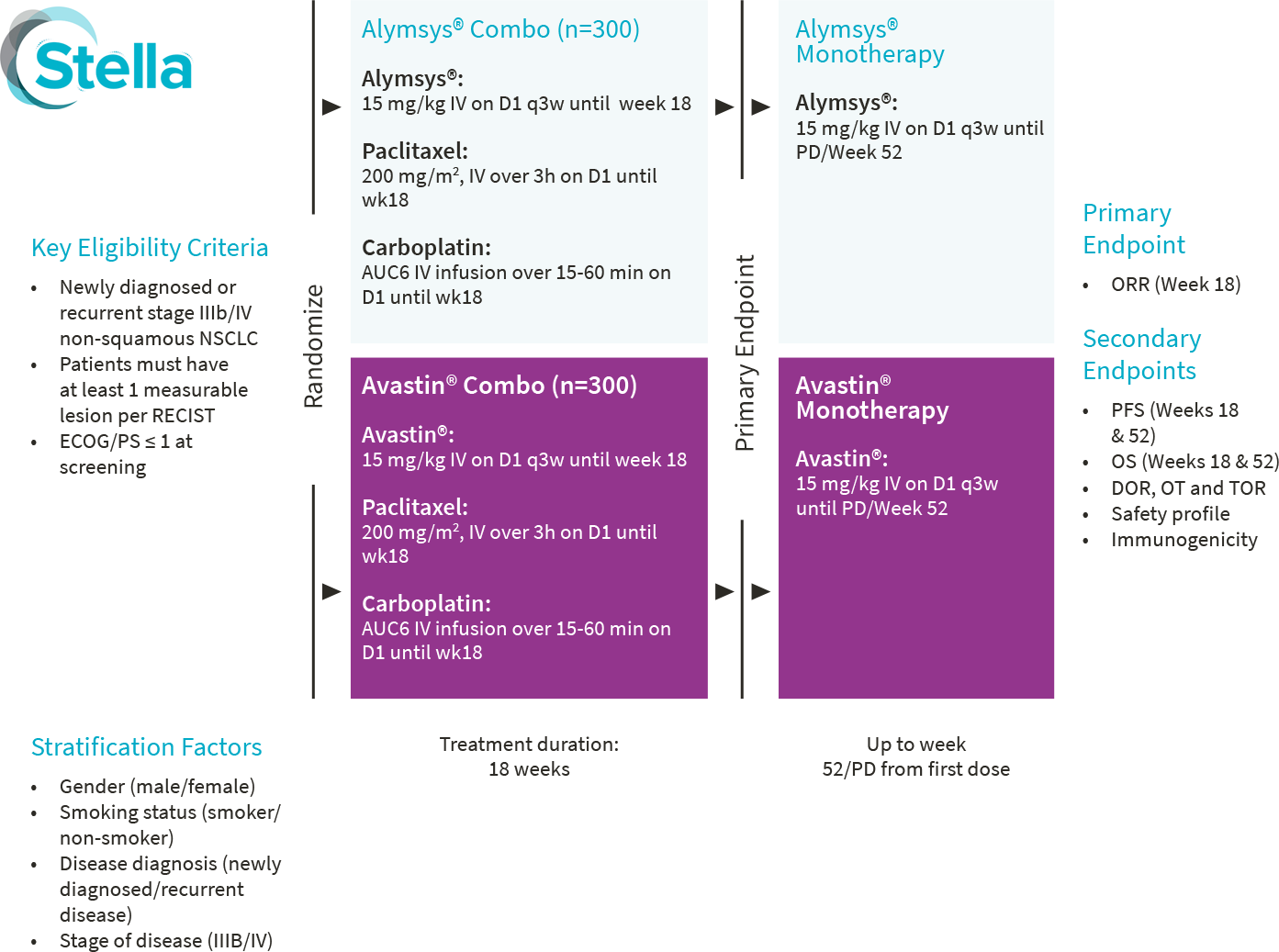
Alymsys®, in combination with carboplatin and paclitaxel, is indicated for the first-line treatment of patients with unresectable, locally advanced, recurrent or metastatic non–squamous non–small cell lung cancer (NSCLC).
Recurrent Glioblastoma (GBM)
7
Alymsys® is indicated for the treatment of recurrent glioblastoma (GBM) in adults.
Metastatic Renal Cell Cancer (mRCC)
7
Alymsys®, in combination with interferon alfa, is indicated for the treatment of metastatic renal cell carcinoma (mRCC).
Persistent, Recurrent, or Metastatic Cervical Cancer
7
Alymsys®, in combination with paclitaxel and cisplatin or paclitaxel and topotecan, is indicated for the treatment of patients with persistent, recurrent, or metastatic cervical cancer.
Epithelial Ovarian, Fallopian tube, or Primary Peritoneal Cancer
7
Alymsys®, in combination with paclitaxel, pegylated liposomal doxorubicin, or topotecan, is indicated for the treatment of patients with platinum-resistant recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer who received no more than 2 prior chemotherapy regimens.